This is how an EFOY direct methanol fuel cell works?
Extraordinarily environmentally friendly
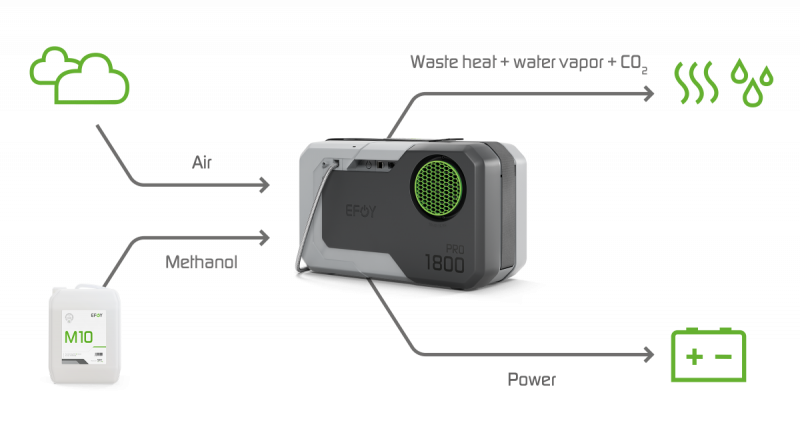
The EFOY Pro charges the batteries fully automatically. The integrated charge controller permanently monitors the charge status of the battery. When required, the EFOY Pro is started automatically and turned off again when the battery has been charged. This means not only will you always have full power reserves, but your batteries will also last much longer because continuous charging prevents deep discharge. 230 V devices can be operated when an inverter is used.
The EFOY fuel cells are based on DMFC (Direct Methanol Fuel Cell) technology. They generate power from the fuel in the fuel cartridge (methanol), complemented by oxygen from the air. Methanol is directly converted into power. In addition to power, all this creates is waste heat and water vapour with a little carbon dioxide. This is extraordinarily environmentally friendly.
The power generating principle of EFOY fuel cells
The EFOY fuel cell converts chemical energy into electrical energy without interim stages, without moving parts and without much loss of efficiency. That is why it is such an efficient power generator. The innovative and patented developments of the EFOY fuel cell enable a miniaturised set-up, high performance, a long service life and a low weight. Using the technology developed in-house, SFC has acquired a unique technological and competitive edge in the field of fuel cell systems for off-grid equipment with the EFOY fuel cells.
The hybrid principle of EFOY fuel cells
Of course, the EFOY fuel cells also work with other power generators. In combination with solar modules, for example, they only turn on when the solar module cannot deliver sufficient power during bad weather.
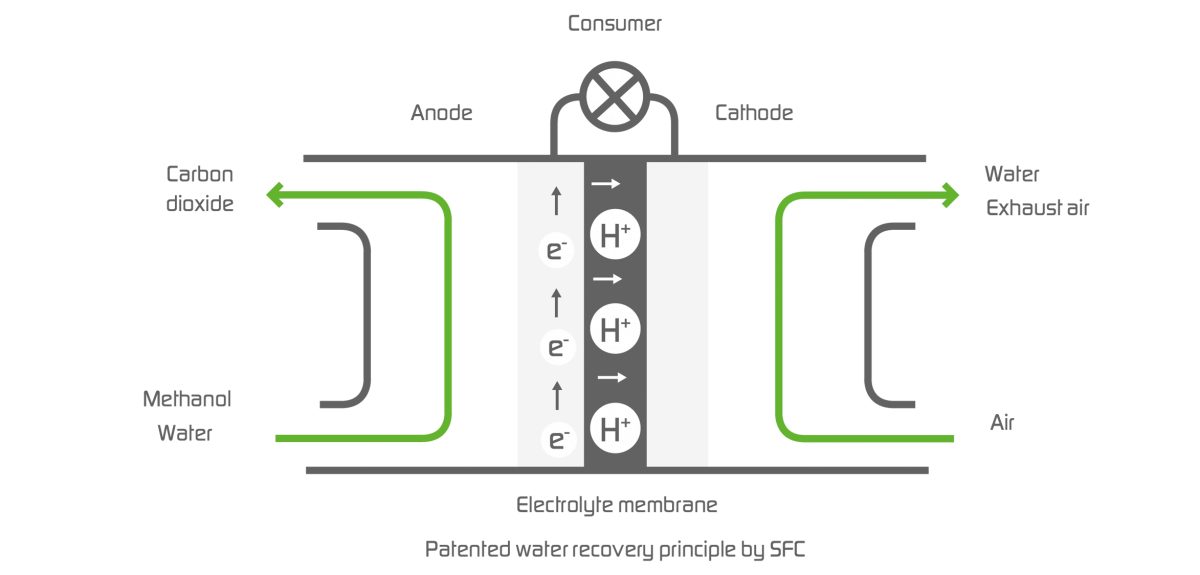
The principle of water recovery
The stack is the power-generating core of the EFOY fuel cells. It consists of individual cells, each of which is set up to include an anode, a cathode and a membrane. The membrane, as an electrolyte, separates anode from cathode. Positively charged, electric particles, so-called protons, can diffuse through the membrane. On the side of the anode, water and methanol are added, on the side of the cathode, oxygen is taken from the ambient air. In the reaction on the anode, H+ ions and free electrons are created, as well as the reaction product carbon dioxide (CO2). The protons can cross the membrane. The electrons, however, have to travel to the cathode side via a connected electric circuit during which they generate power. On the cathode, the H+ ions, the oxygen from the air and the electrons are converted into water vapour. This makes the EFOY fuel cell very environmentally friendly.